
There’s a question I’ve been avoiding:
Does the alkaline pH of Dr. Bronner’s soaps interfere with the acidic pH of the skin’s acid mantle?
It keeps finding me. I knew what answering this would take. I knew that sifting through the hype, marketing, and misinformation surrounding the concepts of the acid mantle, pH balance, and soaps vs. detergents would take a lot of research and a whole lot of time. I was right.
Through the years, I’ve given somewhat glib answers to readers’ questions, mostly along the lines of “don’t worry about it.” I do apologize. Though for the most part, that was – and still is – true, it is hardly a satisfactory answer. In part, I was lazy. In part, I didn’t have the time. In part, I was scared my family’s soap would come up short.
Spoiler alert – it didn’t. I shouldn’t have doubted.
In the spirit of “taking more time to write less” I have extracted the key points from the 17 pages of notes I compiled from reading 40+ studies. You’re welcome. I must admit – once I dove in, it was kind of fun – in a nerdy, geeked-out sort of way. There were times as I read through the research that I yelped for joy. You think I’m kidding?
Now it’s your turn to learn.
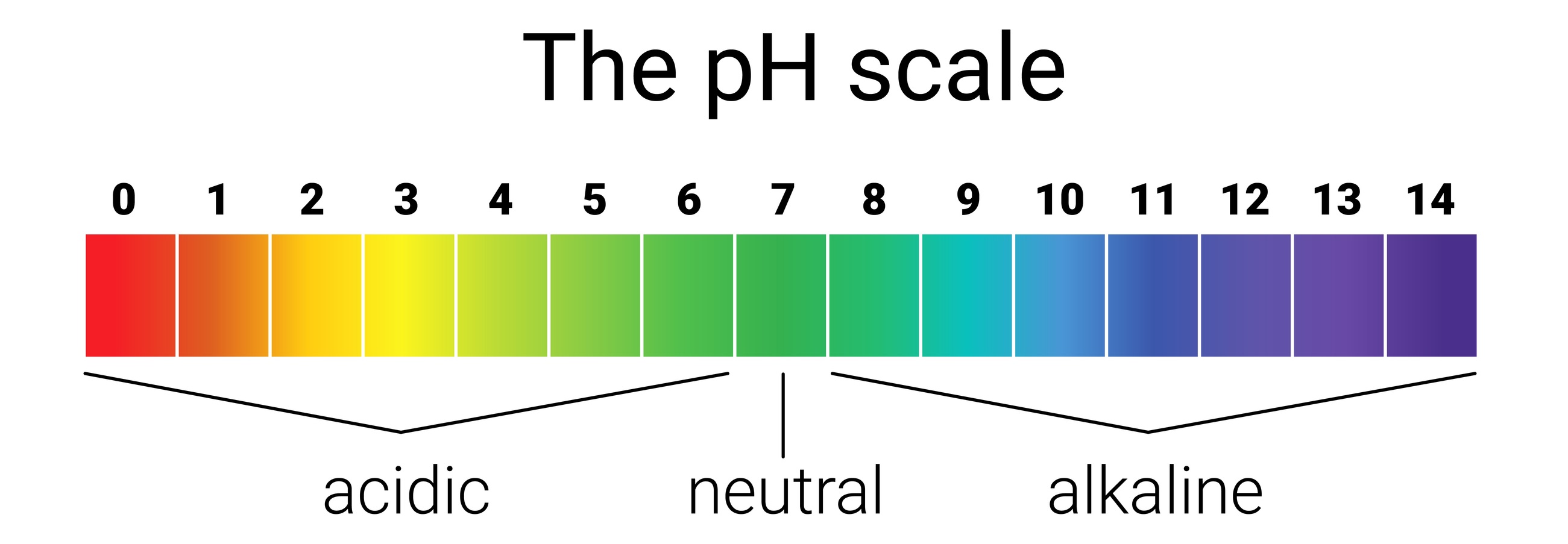
pH
Cast your mind back to 8th grade science when you dipped those little strips of litmus paper into various liquids and the strips instantly turned yellow or purple. That was pH in action. pH stands for “potential Hydrogen” and indicates acidity or alkalinity. Coffee is acidic. Cabbage is alkaline. Water is neutral. After the initial “oohs” and “aahs”, maybe you haven’t thought about it since, but pH is all around you.
Everything that’s water-soluble has pH, measured on a scale from 1 to 14. A neutral pH is in the middle at 7; acids are below 7, and alkalis above 7. Any extreme of pH – towards 1 or 14 – can eat through our skin. Sometimes acids and alkalis react. Think of those baking soda (alkali) & vinegar (acid) volcanoes you made in that same 8th grade science class.
The acid mantle
The acid mantle is a film over our bodies, produced in part by our outermost layer of skin, the Stratum Corneum, and in part by the bacteria that live on our skin. (Now don’t get grossed out by the thought of that bacteria – you need it. It’s your friend.) The acid mantle protects the skin from dehydration, infection, and environmental stressors like wind and grit. To build the acid mantle, the Stratum Corneum contributes sebum (oils, waxes, esters) and sweat (water, salt). The acidity develops when the bacteria on your skin break down the oils into fatty acids and glycerol. The pH of the acid mantle ranges from 5.0 to 5.5.[i]
The pH of skin cleansers
When it comes to skin cleansers, there are two categories: natural soaps and synthetic detergents. Soap has been around for millennia, the simple salt of a fatty acid, made by a one step reaction between an animal fat or plant oil and a strong alkali. Soaps are always alkaline. Milder soaps have a pH around 8-10, while harsher soaps can soar as high as 13. Synthetic detergents, developed in the last century, are derived from more complex reactions based on petroleum or plant oils. They are usually neutral to acidic in their pH.
What this means for Dr. Bronner’s…
Dr. Bronner’s soaps are true soaps made from organic coconut, palm kernel, olive, hemp, and jojoba oils. Because of our careful processes (more below), the pH of the Pure-Castile Liquid Soap is 8.7 – 9.9. We do not have a specific pH range that we can provide for our Pure-Castile Bar Soap, as pH is not traditionally tested in bar soaps. The Organic Sugar Soaps and Organic Shaving Soaps, due to the Shikakai powder, have the even more exceedingly mild pH of 8.0 – 10.0.
The impact of cleansers on the acid mantle
Both soap and detergents impact the acid mantle during cleansing.[ii] Both types of cleansers work in the same way – binding oil to water in a magical process called emulsification. (What a great word!) This means that on our skin, these cleansers are attracted to the oils. This process removes the oils, sweat, bacteria, and odors our body has emitted, as well as any external oils, dirt, bacteria, and grime that has stuck to our skin. Unless we forego washing (which is culturally and hygienically questionable) or wash less (which has some merit in certain cases), the acid mantle is going to get disrupted regularly. But it was built to withstand it. Nonetheless, we need to clean ourselves with as little disruption as possible to the acid mantle and help our skin replenish.
The concern of skin pH and soap
Although cleansing does disrupt the pH of the acid mantle, this disruption is not because of the pH of the cleanser. There is no acid/base reaction like the aforementioned baking soda/vinegar volcano. All cleansers, regardless of their pH, disrupt the pH of the acid mantle – be it an acidic detergent, an alkaline soap, or even neutral water by itself.[iii],[iv] This is because the cleanser is removing some of the acid mantle. Happily, though, the acid mantle is in a constant state of regeneration and returns to its normal pH within 30-90 minutes. The temporary pH disruption is not a concern.[v] Even long-term use of soap does not impact the pH of the acid mantle.[vi]
The import of pH for skin cleansing
To sum it up, pH matters a bit, but not much. As long as you avoid an extreme pH, the skin easily accommodates being washed. In other words, avoiding the extremes, pH does not determine whether a cleanser is good for the skin.[vii] This is a well-documented conclusion, which is drawn in at least 11 distinct studies.[viii] There are more important things to consider in choosing a good cleanser.[ix]
What makes a good cleanser?
Healthy ingredients, for starters.
Regardless what the front of a bottle says about itself, turn the bottle over and read the ingredient list. For help deciphering them, run the list through the Environmental Working Group’s Skin Deep Database to get their hazard rating on it. Look for these red-flags: sodium laureth sulfate, often contaminated by the carcinogenic 1,4-Dioxane;[x] Quaternary compounds, asthmagens that release formaldehyde, another known carcinogen; “Fragrance,” which is a category, not an ingredient, can hide phthalates, linked to reproductive harm as well as a jaw-dropping slew of other maladies.[xi] Problematic preservatives include parabens, which are hormone disruptors, and methylisothiazolinone, a skin irritant. Instead look for ingredients close to nature that receive low hazard ratings from EWG’s database. It also helps if a product has fewer ingredients. You then have less to monitor.
What this means for Dr. Bronner’s…
All Dr. Bronner’s products state a full list of their ingredients clearly on the label. The ingredients are selected to be the healthiest and best for our skin. No cheap shortcuts. The products are all plant based. No synthetics, no petrochemicals, no GMOs. Certified organic ingredients ensure product purity. Any scents are from pure essential oils. No artificial fragrances, no colorants.
Mild surfactants
Mildness is another factor that is more important than pH for maintaining a strong acid mantle and healthy skin. Surfactants, which is a word that describes both soap and detergents, have a tricky job. They are supposed to pick up oils, but how are they to know which oils to take away and which to leave behind? An aggressive surfactant strips out too many oils, even extracting oils below the acid mantle, out of the skin itself, causing irritation.[xii] Leave these cleaners for the dishes, where we want every oil gone. A mild surfactant takes only the readily available oils that are more loosely available on the surface of our skin.
The mildness of a cleanser depends on the size of its molecules. Not that I expect you to have a molecular microscope in your back pocket to see this, but let’s imagine for a moment. Smaller molecules can wiggle their way between skin cells, and have a stronger pull on oils, with the undesirable effect of extracting too many oils out of the skin.[xiii] Larger molecules can’t fit between those skin cells and so leave those oils in place.[xiv] There’s a balance to be found here, though. If a surfactant molecule is too large, it is less water soluble, meaning it doesn’t rinse off well. The size of a soap molecule depends on the length of its “tail,” which is a fatty acid chain. The length of a fatty acid depends on its number of carbon atoms. Soap molecules containing medium-long chain fatty acids (between 12 and 18 carbon atoms) strike this balance perfectly.[xv]
What this means for Dr. Bronner’s…
Fatty acids of this medium-long size are the primary types found in coconut, palm kernel, olive, hemp, and jojoba oils.[xvi] Dr. Bronner’s soaps are made with this perfect blend of oils, making the Pure-Castile and Organic Sugar & Shave Soaps superbly mild. Additionally, Dr. Bronner’s soaps are “superfatted.” This means, in the liquid Castile, Sugar, and Shave Soaps, that after the main soapmaking reaction has finished, a measured amount of citric acid is added to lower the pH and draw out some of the fatty acids. These free fatty acids increase the soap’s mildness, as well as contribute to a creamier lather and smoother feel on the skin. In the Castile Bar Soap, superfatting happens when the hemp seed and jojoba oils are withheld from the saponification reaction and are added afterwards. As I’ll explain below, these two oils have exceptional skin benefits.
Three more ways to strengthen the acid mantle
In addition to minimizing disruption by using mild surfactants, cleansers can further strengthen the acid mantle by incorporating three moisturizing components: occlusives, humectants, and emollients.[xvii] Pull those whoppers out at your next cocktail party! The word nerd inside me is jumping for joy. These three categories aid the skin in unique ways.
Occlusives lie on top of the skin and serve as a substitute acid mantle. Like the acid mantle, they protect against dehydration, infection, and environmental damage, and give the two other types of moisturizers a chance to do their part. Among plant-based ingredients that do this are almond, jojoba, soybean, and avocado oils.[xviii]
Humectants draw moisture back into the skin and work best beneath the protection of an occlusive. Among plant-based ingredients that do this are glycerin[xix] and various sugars, including honey and sucrose.[xx]
Emollients fill in the cracks between skin flakes, replacing lost lipids.[xxi] There are two fatty acids that do this particularly well: linoleic and alpha-linoleic fatty acids, neither of which can the body produce on its own.[xxii] They are readily found in a wide variety nuts, seeds, and leafy vegetables including safflower, wheat germ, sunflower, and hemp oils.[xxiii]
The skin benefits most when all three of these types of moisturizers are present together.
What this means for Dr. Bronners…
All of Dr. Bronner’s soaps contain jojoba oil (an occlusive), glycerin (a humectant), and hemp oil (a concentrated source of linoleic acid, an emollient).[xxiv] This means that the Castile Liquid and Bar Soaps, as well as the Organic Sugar & Shave Soaps supply what our skin needs to be strong and healthy. The Organic Sugar & Shave Soaps contain additional humectants, sucrose (sugar) and white grape juice, resulting in an even more nourishing cleanser.
Conclusion
When I began this self-directed course on pH and skin health, I wanted solid, peer-reviewed, unbiased research. No marketing campaigns. No hype. No trends. No celebrity commentary. I have never researched more than I have for this article. Possibly not even in college. I’ve included many references below for those who cry, “Show me the research!” You are my people, and that is our song. Needless to say, I’m glad to learn that pH of cleansers is almost a non-issue, and that my family’s formulations are well-founded. Though I’ve always thought that, it’s good to know why.
Special thanks to Dr. Vartan Libaridian, Dr. Bronner’s chemistry and R&D consultant, who breaks down the complexities of chemistry into phrases non-chemists can understand, and answered innumerable questions throughout my writing this article.
[i] Oranges, T., Dini, V., & Romanelli, M. (2015). Skin Physiology of the Neonate and Infant: Clinical Implications. Advances in wound care, 4(10), 587-595
[ii] DYKES, P. (1998). Surfactants and the skin. International Journal of Cosmetic Science, 20(1)
[iii] Gfatter R, Hackl P, Braun F: Effects of soap and detergents on skin surface pH, stratum corneum hydration and fat content in infants. Dermatology 1997; 195: 258–262
[iv] Bornkessel, A., Flach, M., Arens‐Corell, M., Elsner, P. and Fluhr, J. W. (2005), Functional assessment of a washing emulsion for sensitive skin: mild impairment of stratum corneum hydration, pH, barrier function, lipid content, integrity and cohesion in a controlled washing test. Skin Research and Technology, 11: 53-60. doi:10.1111/j.1600-0846.2005.00091.x
[v] Bechor R, Zlotogorski A, Dikstein S. Effect of soaps and detergents on the pH and casual lipid levels of the skin surface. J Appl Cosmetol 1988; 6:123-128
[vi] Takagi, Yutaka & Kaneda, K & Miyaki, M & Matsuo, K & Kawada, H & Hosokawa, H. (2014). The long-term use of soap does not affect the pH-maintenance mechanism of human skin. Skin Research and Technology. 21. doi:10.1111/srt.12170
[vii] Mark Oestreicher. Detergents. bath preparations, and other skin cleansers. Clinics in Dermatology 1998; 6:29-36
[viii] Ertel, Keith. (2000). Modern skin cleansers. Dermatologic clinics. 18. 561-75. 10.1016/S0733-8635(05)70207-2
[ix] DYKES, P. (1998). Surfactants and the skin. International Journal of Cosmetic Science, 20(1)
[x] California Environmental Protection Agency (CalEPA), & The Office of Environmental Health Hazard Assessment (OEHHA). (n.d.). 1,4-Dioxane. Retrieved February 18, 2019, from https://oehha.ca.gov/proposition-65/chemicals/14-dioxane
[xi] EWG and the Campaign for Safe Cosmetics. (2010, May). Not So Sexy. Retrieved February 18, 2019, from https://www.ewg.org/research/not-so-sexy
[xii] Draelos ZD. The science behind skincare: Cleansers. J Cosmet Dermatol. 2018; 17:8–14. doi:10.1111/jocd.12469
[xiii]Ertel, Keith. (2000). Modern skin cleansers. Dermatologic clinics. 18. 561-75. 10.1016/S0733-8635(05)70207-2
[xiv] Seweryn, A. (2018). Interactions between surfactants and the skin – Theory and practice. Advances in Colloid and Interface Science, 256, 242-255
[xv] Corazza, M., Lauriola, M. , Zappaterra, M., Bianchi, A. and Virgili, A. (2010), Surfactants, skin cleansing protagonists. Journal of the European Academy of Dermatology and Venereology, 24: 1-6. doi:10.1111/j.1468-3083.2009.03349.x
[xvi] Orsavova, J., Misurcova, L., Ambrozova, J. V., Vicha, R., & Mlcek, J. (2015). Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. International journal of molecular sciences, 16(6), 12871-90. doi:10.3390/ijms160612871
[xvii] Chularojanamontri, L., Tuchinda, P., Kulthanan, K., & Pongparit, K. (2014). Moisturizers for Acne: What are their Constituents?. The Journal of clinical and aesthetic dermatology, 7(5), 36-44.
[xviii] Lin, T. K., Zhong, L., & Santiago, J. L. (2017). Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. International journal of molecular sciences, 19(1), 70. doi:10.3390/ijms19010070
[xix] Ventura, S. A. and Kasting, G. B. (2017), Dynamics of glycerine and water transport across human skin from binary mixtures. Int J Cosmet Sci, 39: 165-178. doi:10.1111/ics.12362
[xx] Burlando, B. and Cornara, L. (2013), Honey in dermatology and skin care: a review. J Cosmet Dermatol, 12: 306-313. doi:10.1111/jocd.12058
[xxi] Chularojanamontri, L., Tuchinda, P., Kulthanan, K., & Pongparit, K. (2014). Moisturizers for Acne: What are their Constituents?. The Journal of clinical and aesthetic dermatology, 7(5), 36-44
[xxii] Khnykin, D., Miner, J. H., & Jahnsen, F. (2011). Role of fatty acid transporters in epidermis: Implications for health and disease. Dermato-endocrinology, 3(2), 53-61
[xxiii] Porto, Carla & Decorti, Deborha & Tubaro, Franco. (2012). Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Industrial Crops and Products. 36. 401–404. 10.1016/j.indcrop.2011.09.015
Further reading
- 5 Life Habits for Healthy Skin
- Benefits of Shikakai for Hair and Body
- Warding Off Dry Skin With Dr. Bronner’s Soaps
This use and many more are in my book, Soap & Soul: A Practical Guide to Minding Your Home, Your Body, and Your Spirit with Dr. Bronner’s Magic Soaps, available now in hardback on DrBronner.com or at your favorite bookseller, and as an eBook and audiobook (read by me!) from wherever you download or listen.








I wanted to know if it was mild enough to clean my antique marble table top. I finally found the answer! Thanks for the info!!!
Hi Katy- You’re so welcome! Glad this helpful!
Would adding mandelic acid to the peppermint soap be extremely detrimental to the integrity of the soap?
Hi Carmen- While I’ve not personally tested this, I strongly suspect the low pH of the Mandelic acid would cause acid/base reaction with the alkaline Castile Soap to the extent that the soap would become unsaponified – or reverted back to their original oils.
what about sodium hydroxide? I read it is very drying and you did not mention it in your article. thanks
Hi Andrea- You’re giving me a chance to talk about soap chemistry, which I always enjoy! Soap is made, and has been made for millennia, by combining oils with strong alkalis. In our case, for the oils we use a blend of coconut, olive, palm (or palm kernel), hemp and jojoba. For the alkali we use sodium hydroxide. When the oil and alkali gets together, all the molecules come apart. The fatty acid sections from the oil molecule recombines with the sodium ion from the alkali and forms a salt of a fatty acid – more commonly known as soap. And then those hydroxide sections of the alkali combine with each other and form water. We test every batch to make sure all those alkali molecules got consumed so that there is none left in the final product. You can read more about the chemistry behind it here: https://www.drbronner.com/blogs/our-customers/making-the-best-soap
Can Dr. Bronner’s soap be used as a base for shampoo?
I was told the PH is too high.
If it is, what can be added to bring it down?
Thank you.
Hi Kelli – I have washed my hair with Dr. Bronner’s Castile or Sugar soaps for over 10 years now! You can read more about my transition from shampoo to soap in my article here. You are right that pH is a factor. Our hair is smoothest when rinsed with an acid. Since soap is alkaline, it is important to follow up with an acidic rinse, as I discuss in the article I linked. You cannot lower the pH of soap without destroying the soap molecule, so best to go with the option of an acidic rinse afterwards.
Another great resource is my colleague Rafi Loiderman’s article A Definitive Guide to Using Dr. Bronner’s as a Natural Shampoo.
because you wrote this article i will give the baby castille soap a try. thank you so very much!!!!!
also i don’t understand the purpose of sugar soap… sorry. i tried real hard to understand the difference.
Hi Janet- The main difference is that in that in place of water, the Organic Sugar Soap Castile Soap is made organic white grape juice, organic sucrose, and organic Shikakai powder. These three ingredients are humectants – that means they draw moisture into the skin, and as such, make the Organic Sugar Soap a more moisturizing soap option for people with very dry skin. For more on the difference between Castile Soap and Organic Sugar Soap, take a look at my blog post, https://www.lisabronner.com/dr-bronners-pure-castile-vs-shikakai-soaps/
thank you for this information. i love learning. i am a nerd and it is important for me to understand why something is working or not working. thank you so very much…..seriously.
I’m so glad this resonated with you, Janet!
Our home was exposed to a corrosive cleaning compound that cleans front loading washing machines. I cannot use hydrogen peroxide to take care of it, I’m sensitive to it. I’m looking for a neutral cleaner to use as close to water as possible. I’ve been warned that foams are acidic and may react with the bleach in the compound. Do you have data on which of your soaps are at what level on the alkalinity/base scale so I can choose one closest to neutral to help clean up this mess?
Hi EB- The cleanser we have that is closest to neutral for this sort of cleaning is our Pure-Castile Liquid soap. It has a pH of 9.3.
I was always taught that the lower pH range (acidic) is better to use, because it doesn’t mess with the pH range of the mantle of your skin. If you use a higher pH in the alkaline range, you will notice that your private parts and your armpits will get stinky faster. The reason for that is because you have taken it out of the lower pH range and it has killed the good bacteria along with everything else. I would love to know your thoughts about this.
Additionally, the higher alkalinity soaps will burn more if you get them in a cut or your eyes. You might call it mild, but it’s not when it comes to delicate things…
Hi Rie- Any cleanser regardless of pH will wash off the acid mantle. Whether the cleanser is acidic or alkaline, they all use the power of micelle formation to remove the acid mantle. However, the acid mantle is in a constant state of regeneration, and so it is quickly back in place. No cleanser can change the pH of the skin itself. As far as odor goes, other factors such as what we eat are much more impactful. A more plant-based diet is known to reduce body odor.
I cannot express how unbelievably relieved I am to have this knowledge as well as all the articles’ knowledge that you post here on this site. I love knowing ALL the research and facts and am right there “nerding” alongside you. Chemistry is fascinating and knowing the why behind things can help us consumers to make wiser choices that will ultimately and hopefully change store shelves across the nation! Love that my family and I can now feel relaxed to trust the Dr. Bronner’s products for our generations to come. Thank you for continuing to take the time to dive into these topics.
Hi Britni- I’m so glad this resonated with you!
Thank you, thank you, thank you, thank you for doing this!!!!!!!!!
I have myself been walking around this question without daring to jump in. Stumpled across you article and I feel very thankfull for all the work you put into this 🙂
I’m so glad to hear you found this article helpful, Stine!
Thank you for this. I’ve never questioned your products and raised my vegetarians vegan girls with your soaps. Exit stage left…a breast cancer diagnosis has had more paperwork given to me which leaves unanswered questions. So as I get ready to start radiation the paperwork suggests a pH balanced soap. Your paper has cleared this up with zero doubts that I continue with your products.
Hi Lisa- I’m so sorry to hear about your diagnosis and wish you all the best during your treatment. I’m glad this article was helpful to you.
Thank God that I continued to research Dr Bronners soap after reading some very negative reviews regarding the pH. Sometimes the internet is like looking at the world with one eye closed. You put in the time, you listed your sources, you made a compelling argument and you put my thinking back on the correct track. I will continue to use a product family that I’ve enjoyed since starting college in 1968….Shine ON 🌞🌛
Thanks, Marshall! Glad this was helpful! I am so curious, where did you find the product in 1968?
Appreciate the researched response. The problem defenders make is not understanding that research and what is purported is based on a healthy model and not on clinical reality. People who suffer chronic illnesses, immune dysfunction, environmental illness, chronic inflammatory syndromes, gut dysbiosis, etc. are NOT healthy. These are the very people who are likely to be using natural, fragrance-free products.
When people ask the pH of such products, they may have already had a bad experience from other natural products that they determined to be related to pH.
Years ago after developing multiple chemical sensitivities and tiring of Vanicream, I experimented with various brabds of natural castille soaps. I was using these soaps on my entire body. I developed bacterial vaginosis, which I had never had before. Months later, I got it again after trying another soap. It coincided with my use of natural soaps. I went back to Vanicream and no more BV. I had read an enlightening comment pertaining to pH online in a review of a particular natural soap I was looking at. The light bulb went off, and I then understood that the soap I had used had somehow altered the microbial balance in my vagina, resulting in the bacterial infection.
Since then, I have been mindful not to use natural soaps in the shower. I have been using Bronner’s liquid soap recently on face, hands, arms, with caution. I am aware that Staph lives on the skin, so alterations to the skin microbiome in a person who is unhealthy and/or on certain medications has the potential to be problematic.
Thanks for the great reseach!
I have a question: What about the Dr. Bronner lotions? Do they also contain humectants, occlusives and emollients?
Because I am not shure about the humectant (glycerin)… I can’t find that in the ingredient List for the moistruizer.
I wanne be shure that the lotions are really moistruizing! How do you do that with the few ingrediences… With what do oil and water bind together? (If you dont use the chemical ‘normal’ ingredients)
I would really love to get an answer 🙂
Hi Lisa- Thanks for taking such a close look at our ingredients! In our Organic Lotion, Jojoba oil is an occlusive, while Coconut, Jojoba and Hemp Seed oils are all emollient. Our lotion doesn’t contain a humectant, but adding one is currently under consideration. Ethanol, which is derived from certified non-GMO sugarcane, is what holds all the moisturizing oils together in suspension. This is called a solubilizer. Without it, the oils in the lotion would separate. The level of ethanol in the lotion is balanced with other ingredients to make sure that it doesn’t dry the skin, and because it holds the other ingredients together, it actually helps the Lotion be more moisturizing.
I’m writing because Dr.Bronner’s (baby scent) exacerbated eczema… and so did all the nice good old natural soaps like marseille….
I went back to using Aveeno moisturizing bar (pH 4.8) and got much better.
Hi Sun- It’s great you found something that works for you! Moisturization is the key to managing eczema. When it comes to using Dr. Bronner’s, some customers have more success with our most moisturizing soaps, which starting from the most moisturizing are: Shaving Soap, Sugar Soap, Bar Soap. Liquid Soap is our least moisturizing and does not work for all who suffer with eczema.
The problem is, people with eczema/psoriasis/sensitive skin often do not have healthy skin that returns to its normal pH after using soap
Hi Sun- The specific soap (and its pH) is less important than the Soak and Seal method: moisturize with a balm or lotion no more than 3 minutes after stepping out of the bath or shower.
Wow, great read! I often put things off knowing full well they will take plenty of precious time. I enjoyed this “nerdy” read and although I have only used one of your products, I am now excited to try more because I feel like I “know the person behind the product” and that means a lot! Thanks for doing this research Lisa!
Thanks for reading, Rachel!
Thanks for these details! I’m guessing the same is true for our dogs? I had used the lavender or unscented to bathe my dog. I was scolded by a friend telling me it was totally the wrong ph. I’ve not found a good “pure” dog product that I like as well!
Hi Carolyn- While human skin has an acidic pH, dog skin is generally found to be alkaline. Because Castile Soap is also alkaline, they do “match.” Keep in mind too that a surfactant (or cleaning agent) can have the exact same pH as a dog’s skin, yet be far too aggressive and cause irritation by stripping the skin of needed oils. Their skin is not as impacted by pH as by the aggressiveness of the cleaning agent. (As you know from this article, this is true of people skin as well.) The surfactants in the Castile soap – saponified olive/coconut/palm oils – are mild, non-aggressive cleansers that gently remove dirt and grime without stripping the skin of its needed oils. Just ask my black Lab, Tucker! (https://www.lisabronner.com/dog-washing-with-dr-bronners-soap/)
What a good read- thanks for your research Lisa. I will continue to use your family’s soap for all my needs!