
There’s a question I’ve been avoiding:
Does the alkaline pH of Dr. Bronner’s soaps interfere with the acidic pH of the skin’s acid mantle?
It keeps finding me. I knew what answering this would take. I knew that sifting through the hype, marketing, and misinformation surrounding the concepts of the acid mantle, pH balance, and soaps vs. detergents would take a lot of research and a whole lot of time. I was right.
Through the years, I’ve given somewhat glib answers to readers’ questions, mostly along the lines of “don’t worry about it.” I do apologize. Though for the most part, that was – and still is – true, it is hardly a satisfactory answer. In part, I was lazy. In part, I didn’t have the time. In part, I was scared my family’s soap would come up short.
Spoiler alert – it didn’t. I shouldn’t have doubted.
In the spirit of “taking more time to write less” I have extracted the key points from the 17 pages of notes I compiled from reading 40+ studies. You’re welcome. I must admit – once I dove in, it was kind of fun – in a nerdy, geeked-out sort of way. There were times as I read through the research that I yelped for joy. You think I’m kidding?
Now it’s your turn to learn.
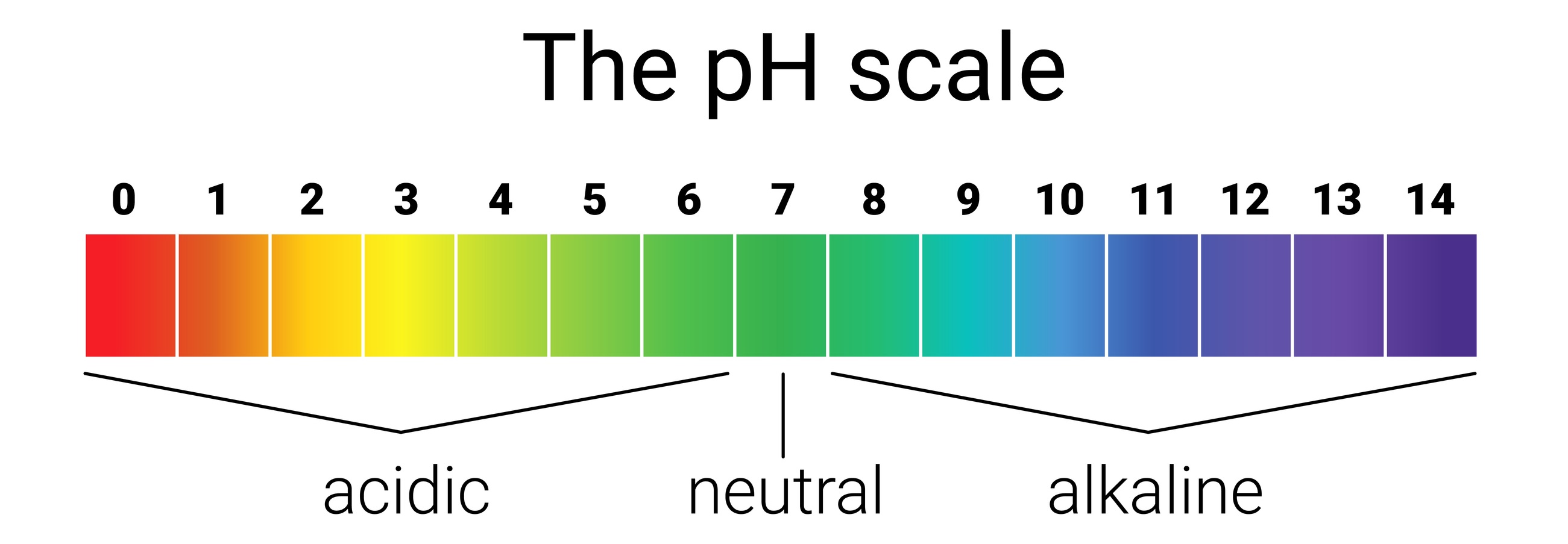
pH
Cast your mind back to 8th grade science when you dipped those little strips of litmus paper into various liquids and the strips instantly turned yellow or purple. That was pH in action. pH stands for “potential Hydrogen” and indicates acidity or alkalinity. Coffee is acidic. Cabbage is alkaline. Water is neutral. After the initial “oohs” and “aahs”, maybe you haven’t thought about it since, but pH is all around you.
Everything that’s water-soluble has pH, measured on a scale from 1 to 14. A neutral pH is in the middle at 7; acids are below 7, and alkalis above 7. Any extreme of pH – towards 1 or 14 – can eat through our skin. Sometimes acids and alkalis react. Think of those baking soda (alkali) & vinegar (acid) volcanoes you made in that same 8th grade science class.
The acid mantle
The acid mantle is a film over our bodies, produced in part by our outermost layer of skin, the Stratum Corneum, and in part by the bacteria that live on our skin. (Now don’t get grossed out by the thought of that bacteria – you need it. It’s your friend.) The acid mantle protects the skin from dehydration, infection, and environmental stressors like wind and grit. To build the acid mantle, the Stratum Corneum contributes sebum (oils, waxes, esters) and sweat (water, salt). The acidity develops when the bacteria on your skin break down the oils into fatty acids and glycerol. The pH of the acid mantle ranges from 5.0 to 5.5.[i]
The pH of skin cleansers
When it comes to skin cleansers, there are two categories: natural soaps and synthetic detergents. Soap has been around for millennia, the simple salt of a fatty acid, made by a one step reaction between an animal fat or plant oil and a strong alkali. Soaps are always alkaline. Milder soaps have a pH around 8-10, while harsher soaps can soar as high as 13. Synthetic detergents, developed in the last century, are derived from more complex reactions based on petroleum or plant oils. They are usually neutral to acidic in their pH.
What this means for Dr. Bronner’s…
Dr. Bronner’s soaps are true soaps made from organic coconut, palm kernel, olive, hemp, and jojoba oils. Because of our careful processes (more below), the pH of the Pure-Castile Liquid Soap is 8.7 – 9.9. We do not have a specific pH range that we can provide for our Pure-Castile Bar Soap, as pH is not traditionally tested in bar soaps. The Organic Sugar Soaps and Organic Shaving Soaps, due to the Shikakai powder, have the even more exceedingly mild pH of 8.0 – 10.0.
The impact of cleansers on the acid mantle
Both soap and detergents impact the acid mantle during cleansing.[ii] Both types of cleansers work in the same way – binding oil to water in a magical process called emulsification. (What a great word!) This means that on our skin, these cleansers are attracted to the oils. This process removes the oils, sweat, bacteria, and odors our body has emitted, as well as any external oils, dirt, bacteria, and grime that has stuck to our skin. Unless we forego washing (which is culturally and hygienically questionable) or wash less (which has some merit in certain cases), the acid mantle is going to get disrupted regularly. But it was built to withstand it. Nonetheless, we need to clean ourselves with as little disruption as possible to the acid mantle and help our skin replenish.
The concern of skin pH and soap
Although cleansing does disrupt the pH of the acid mantle, this disruption is not because of the pH of the cleanser. There is no acid/base reaction like the aforementioned baking soda/vinegar volcano. All cleansers, regardless of their pH, disrupt the pH of the acid mantle – be it an acidic detergent, an alkaline soap, or even neutral water by itself.[iii],[iv] This is because the cleanser is removing some of the acid mantle. Happily, though, the acid mantle is in a constant state of regeneration and returns to its normal pH within 30-90 minutes. The temporary pH disruption is not a concern.[v] Even long-term use of soap does not impact the pH of the acid mantle.[vi]
The import of pH for skin cleansing
To sum it up, pH matters a bit, but not much. As long as you avoid an extreme pH, the skin easily accommodates being washed. In other words, avoiding the extremes, pH does not determine whether a cleanser is good for the skin.[vii] This is a well-documented conclusion, which is drawn in at least 11 distinct studies.[viii] There are more important things to consider in choosing a good cleanser.[ix]
What makes a good cleanser?
Healthy ingredients, for starters.
Regardless what the front of a bottle says about itself, turn the bottle over and read the ingredient list. For help deciphering them, run the list through the Environmental Working Group’s Skin Deep Database to get their hazard rating on it. Look for these red-flags: sodium laureth sulfate, often contaminated by the carcinogenic 1,4-Dioxane;[x] Quaternary compounds, asthmagens that release formaldehyde, another known carcinogen; “Fragrance,” which is a category, not an ingredient, can hide phthalates, linked to reproductive harm as well as a jaw-dropping slew of other maladies.[xi] Problematic preservatives include parabens, which are hormone disruptors, and methylisothiazolinone, a skin irritant. Instead look for ingredients close to nature that receive low hazard ratings from EWG’s database. It also helps if a product has fewer ingredients. You then have less to monitor.
What this means for Dr. Bronner’s…
All Dr. Bronner’s products state a full list of their ingredients clearly on the label. The ingredients are selected to be the healthiest and best for our skin. No cheap shortcuts. The products are all plant based. No synthetics, no petrochemicals, no GMOs. Certified organic ingredients ensure product purity. Any scents are from pure essential oils. No artificial fragrances, no colorants.
Mild surfactants
Mildness is another factor that is more important than pH for maintaining a strong acid mantle and healthy skin. Surfactants, which is a word that describes both soap and detergents, have a tricky job. They are supposed to pick up oils, but how are they to know which oils to take away and which to leave behind? An aggressive surfactant strips out too many oils, even extracting oils below the acid mantle, out of the skin itself, causing irritation.[xii] Leave these cleaners for the dishes, where we want every oil gone. A mild surfactant takes only the readily available oils that are more loosely available on the surface of our skin.
The mildness of a cleanser depends on the size of its molecules. Not that I expect you to have a molecular microscope in your back pocket to see this, but let’s imagine for a moment. Smaller molecules can wiggle their way between skin cells, and have a stronger pull on oils, with the undesirable effect of extracting too many oils out of the skin.[xiii] Larger molecules can’t fit between those skin cells and so leave those oils in place.[xiv] There’s a balance to be found here, though. If a surfactant molecule is too large, it is less water soluble, meaning it doesn’t rinse off well. The size of a soap molecule depends on the length of its “tail,” which is a fatty acid chain. The length of a fatty acid depends on its number of carbon atoms. Soap molecules containing medium-long chain fatty acids (between 12 and 18 carbon atoms) strike this balance perfectly.[xv]
What this means for Dr. Bronner’s…
Fatty acids of this medium-long size are the primary types found in coconut, palm kernel, olive, hemp, and jojoba oils.[xvi] Dr. Bronner’s soaps are made with this perfect blend of oils, making the Pure-Castile and Organic Sugar & Shave Soaps superbly mild. Additionally, Dr. Bronner’s soaps are “superfatted.” This means, in the liquid Castile, Sugar, and Shave Soaps, that after the main soapmaking reaction has finished, a measured amount of citric acid is added to lower the pH and draw out some of the fatty acids. These free fatty acids increase the soap’s mildness, as well as contribute to a creamier lather and smoother feel on the skin. In the Castile Bar Soap, superfatting happens when the hemp seed and jojoba oils are withheld from the saponification reaction and are added afterwards. As I’ll explain below, these two oils have exceptional skin benefits.
Three more ways to strengthen the acid mantle
In addition to minimizing disruption by using mild surfactants, cleansers can further strengthen the acid mantle by incorporating three moisturizing components: occlusives, humectants, and emollients.[xvii] Pull those whoppers out at your next cocktail party! The word nerd inside me is jumping for joy. These three categories aid the skin in unique ways.
Occlusives lie on top of the skin and serve as a substitute acid mantle. Like the acid mantle, they protect against dehydration, infection, and environmental damage, and give the two other types of moisturizers a chance to do their part. Among plant-based ingredients that do this are almond, jojoba, soybean, and avocado oils.[xviii]
Humectants draw moisture back into the skin and work best beneath the protection of an occlusive. Among plant-based ingredients that do this are glycerin[xix] and various sugars, including honey and sucrose.[xx]
Emollients fill in the cracks between skin flakes, replacing lost lipids.[xxi] There are two fatty acids that do this particularly well: linoleic and alpha-linoleic fatty acids, neither of which can the body produce on its own.[xxii] They are readily found in a wide variety nuts, seeds, and leafy vegetables including safflower, wheat germ, sunflower, and hemp oils.[xxiii]
The skin benefits most when all three of these types of moisturizers are present together.
What this means for Dr. Bronners…
All of Dr. Bronner’s soaps contain jojoba oil (an occlusive), glycerin (a humectant), and hemp oil (a concentrated source of linoleic acid, an emollient).[xxiv] This means that the Castile Liquid and Bar Soaps, as well as the Organic Sugar & Shave Soaps supply what our skin needs to be strong and healthy. The Organic Sugar & Shave Soaps contain additional humectants, sucrose (sugar) and white grape juice, resulting in an even more nourishing cleanser.
Conclusion
When I began this self-directed course on pH and skin health, I wanted solid, peer-reviewed, unbiased research. No marketing campaigns. No hype. No trends. No celebrity commentary. I have never researched more than I have for this article. Possibly not even in college. I’ve included many references below for those who cry, “Show me the research!” You are my people, and that is our song. Needless to say, I’m glad to learn that pH of cleansers is almost a non-issue, and that my family’s formulations are well-founded. Though I’ve always thought that, it’s good to know why.
Special thanks to Dr. Vartan Libaridian, Dr. Bronner’s chemistry and R&D consultant, who breaks down the complexities of chemistry into phrases non-chemists can understand, and answered innumerable questions throughout my writing this article.
[i] Oranges, T., Dini, V., & Romanelli, M. (2015). Skin Physiology of the Neonate and Infant: Clinical Implications. Advances in wound care, 4(10), 587-595
[ii] DYKES, P. (1998). Surfactants and the skin. International Journal of Cosmetic Science, 20(1)
[iii] Gfatter R, Hackl P, Braun F: Effects of soap and detergents on skin surface pH, stratum corneum hydration and fat content in infants. Dermatology 1997; 195: 258–262
[iv] Bornkessel, A., Flach, M., Arens‐Corell, M., Elsner, P. and Fluhr, J. W. (2005), Functional assessment of a washing emulsion for sensitive skin: mild impairment of stratum corneum hydration, pH, barrier function, lipid content, integrity and cohesion in a controlled washing test. Skin Research and Technology, 11: 53-60. doi:10.1111/j.1600-0846.2005.00091.x
[v] Bechor R, Zlotogorski A, Dikstein S. Effect of soaps and detergents on the pH and casual lipid levels of the skin surface. J Appl Cosmetol 1988; 6:123-128
[vi] Takagi, Yutaka & Kaneda, K & Miyaki, M & Matsuo, K & Kawada, H & Hosokawa, H. (2014). The long-term use of soap does not affect the pH-maintenance mechanism of human skin. Skin Research and Technology. 21. doi:10.1111/srt.12170
[vii] Mark Oestreicher. Detergents. bath preparations, and other skin cleansers. Clinics in Dermatology 1998; 6:29-36
[viii] Ertel, Keith. (2000). Modern skin cleansers. Dermatologic clinics. 18. 561-75. 10.1016/S0733-8635(05)70207-2
[ix] DYKES, P. (1998). Surfactants and the skin. International Journal of Cosmetic Science, 20(1)
[x] California Environmental Protection Agency (CalEPA), & The Office of Environmental Health Hazard Assessment (OEHHA). (n.d.). 1,4-Dioxane. Retrieved February 18, 2019, from https://oehha.ca.gov/proposition-65/chemicals/14-dioxane
[xi] EWG and the Campaign for Safe Cosmetics. (2010, May). Not So Sexy. Retrieved February 18, 2019, from https://www.ewg.org/research/not-so-sexy
[xii] Draelos ZD. The science behind skincare: Cleansers. J Cosmet Dermatol. 2018; 17:8–14. doi:10.1111/jocd.12469
[xiii]Ertel, Keith. (2000). Modern skin cleansers. Dermatologic clinics. 18. 561-75. 10.1016/S0733-8635(05)70207-2
[xiv] Seweryn, A. (2018). Interactions between surfactants and the skin – Theory and practice. Advances in Colloid and Interface Science, 256, 242-255
[xv] Corazza, M., Lauriola, M. , Zappaterra, M., Bianchi, A. and Virgili, A. (2010), Surfactants, skin cleansing protagonists. Journal of the European Academy of Dermatology and Venereology, 24: 1-6. doi:10.1111/j.1468-3083.2009.03349.x
[xvi] Orsavova, J., Misurcova, L., Ambrozova, J. V., Vicha, R., & Mlcek, J. (2015). Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. International journal of molecular sciences, 16(6), 12871-90. doi:10.3390/ijms160612871
[xvii] Chularojanamontri, L., Tuchinda, P., Kulthanan, K., & Pongparit, K. (2014). Moisturizers for Acne: What are their Constituents?. The Journal of clinical and aesthetic dermatology, 7(5), 36-44.
[xviii] Lin, T. K., Zhong, L., & Santiago, J. L. (2017). Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. International journal of molecular sciences, 19(1), 70. doi:10.3390/ijms19010070
[xix] Ventura, S. A. and Kasting, G. B. (2017), Dynamics of glycerine and water transport across human skin from binary mixtures. Int J Cosmet Sci, 39: 165-178. doi:10.1111/ics.12362
[xx] Burlando, B. and Cornara, L. (2013), Honey in dermatology and skin care: a review. J Cosmet Dermatol, 12: 306-313. doi:10.1111/jocd.12058
[xxi] Chularojanamontri, L., Tuchinda, P., Kulthanan, K., & Pongparit, K. (2014). Moisturizers for Acne: What are their Constituents?. The Journal of clinical and aesthetic dermatology, 7(5), 36-44
[xxii] Khnykin, D., Miner, J. H., & Jahnsen, F. (2011). Role of fatty acid transporters in epidermis: Implications for health and disease. Dermato-endocrinology, 3(2), 53-61
[xxiii] Porto, Carla & Decorti, Deborha & Tubaro, Franco. (2012). Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Industrial Crops and Products. 36. 401–404. 10.1016/j.indcrop.2011.09.015
Further reading
- 5 Life Habits for Healthy Skin
- Benefits of Shikakai for Hair and Body
- Warding Off Dry Skin With Dr. Bronner’s Soaps
This use and many more are in my book, Soap & Soul: A Practical Guide to Minding Your Home, Your Body, and Your Spirit with Dr. Bronner’s Magic Soaps, available now in hardback on DrBronner.com or at your favorite bookseller, and as an eBook and audiobook (read by me!) from wherever you download or listen.








Wow! That was a great “all I’ve ever wanted to know” about soap article. Really appreciate your research and ability to share it in a way I can understand!
A new pH question–
We drain water from a walk-in tub, into a system that sustains a garden bed.
We use your Liquid soaps in the bathtub and love them.
QUESTION — Will the Alkaline pH of your soap damage plants?
Some plants need acid pH, many prefer neutral pH, only a few like alkaline pH.
I don’t expect you to launch a whole new research project–just wonder whether you have encountered this issue, or maybe you could suggest somebody else who would be more likely to know about this issue
Hi there- I imagine the amount of soap you’d use in the tub is relatively small. Add that to that fact that its highly diluted in the tub water, and it’s not enough to change the pH of the soil significantly.
Thank you for this information. I love Dr Bonner’s soaps I have been using them for over a year now and my skin is looking great. I was breaking out due to having to wear a mask all the time and the soap has cleared that up. Plus, I love washing my mask in the scented, not only the scented soap makes them smell wonderful, but it cleans the makeup right off the mask.
Also, when I had covid the organic magic menthol balm was a life saver. I used it on my chest, back and joints, it works wonders! I really enjoy all the products I have tried, and I’m very happy with the products.
Hi Karen- It’s great to hear our products work well for you! Thank you for sharing!
Thank you so much for the time you invested. I am new in the work of soap but i want to learn how take care of my skin, health and the plante. Your summary is easy to read !!!
This a wonderfully written and informative article. 5 Stars! Thank you for this information.
Wow!! I really enjoyed your article. I found it to be well written, well researched (I looked at the reference studies and for the most part found them to be well conducted and managed) and easy to understand. Thank you for taking the time to locate, study, condense and present this information. I will continue using your products.
Hi Valerie- Thank you for your kind words. It’s great to hear you found the research helpful!
Thank you so much for this information. I’ve used Dr. Bronner’s liquid soaps for both shower and
shampoo for decades just because they have no ‘chemicals’ and long, multisyllable ingredients, plus
they smell good (peppermint) and feel good. But I have wondered about the pH issue. 40 years of
Dr. Bronner’s and so far, so good. I really appreciate your respect for and commitment to your
customers.
Thank you, Judith. Its always a pleasure to hear from our long-time users.
Hi Lisa,
I’ve been using your castile soap for sometime now and I love it, I also recently started to use Sal Suds. That is now my favorite all time dish soap, I have very sensitive skin and now that I’m retired I just wash up the dishes during the day as we use them. Well Dawn dish soap was drying my hands out so badly I had to switch. Many other brands don’t cut the grease as well as Dawn, then along came Sal Suds, it does the job, and has a light fresh scent, even though it says unscented in the descriptions. The scent is so light it hasn’t bothered my allergies so I’m keeping it.
Hi Karen- Excellent! Glad to hear Sal Suds is working for you. It’s good for a whole lot more than dishes too. If you haven’t already, check out the Sal Suds Dilutions Cheat Sheet: https://www.lisabronner.com/sal-suds-dilution-cheat-sheet/
Lisa, You did a terrific job with this article. In fact you made it interesting. Considering the topic, it takes a good writer.
I too am a bit of a nerd so can’t resist the following note for emphasis even though you make it clear: Water is the solvent, surfactants help water do the job. Thorough rinsing is the way to clean. For example, scrubbing hands for more than 20 seconds was emphasized to reduce the spread of COVID 19 and yet the importance of thorough rinsing was not.
Thanks, again.
What is the ph level of sal suds?
Hi Quincy- The pH of Sal Suds, which is our household cleaner, is 11.0–11.5.
Hi there Lisa, I was just wondering is it okay to use your soap in combination with other body washes like a hydrating body wash? Also can I add more oil to your soap to make it more moisturizing? I feel very dry after using your tea tree soap but it’s the only thing I’ve found that helps me with acne on my face and body. I stopped using your soap for a couple weeks and I broke out like crazy. Started using it again and have been clearing back up. I’m just trying to tweak the soap a bit to feel hydrated after using it.
Hi Gio- It’s great to hear you’re finding the Tea Tree so helpful! Soap’s job, in part, is to bond to oils. It does not differentiate between added oils or oils on your body. Adding too much oil to the soap will busy the soap with chasing soap molecules and not leave any left for chasing down the grime on hands and skin. Rather than adding to the liquid Castile, try either the Castile Bar Soap or Organic Sugar Soap, both of which hydrate dryer skin. And both come in Tea Tree! I cover some of the differences in the soaps in this blog post: https://www.lisabronner.com/warding-off-dry-skin-with-dr-bronners-soaps/
Hi. Could you be more specific about what extremes are in PH? (i.e. what PH level/number). I’m in Asia and bought a liquid soap that claims that it’s gentle because of its PH level. I tested the soap with a PH strip and it has a PH of just 3. Is that too low? Too acidic?
Hi Eric- You may notice I responded previously, but a few comments were lost, and your question may help others. A pH of 3 would be about the equivalent of orange juice or soda. Not too harsh. However, what’s more important than pH in skincare products is the intensity of the surfactant, which is the agent that is actually doing the cleaning. Take a look at the ingredient list and see what you have there. You can send me the top five ingredients to look at or run them through the Environmental Working Group’s Skin Deep Database. You might even find the product listed there.
Well, thank you for the very detailed and clear answer! Today I had the first shower in my life with Dr.Bronner liquid castille soap. It’s amazing, the work you do. I couldn’t believe such a quality and combination of ingredients exist with a reasonable price. I noticed a bit dry skin after the shower so wanted to check about Ph of the soap, though maybe it’s a bit too alkaline like the old natural soaps used to be. But now I got the explanation, thank you, Lisa.
The only question I got is about hair. It is a bit oily after washing. On the bottle it is written that you’re supposed to use a conditioner like smth with vinegar. Where can I read how exactly I can make it?
Hi Veronica- Excellent! For an acidic rinse I use a dilution of half apple cider vinegar and half water. Rinse shampoo from hair, pour rinse over hair, then rinse out. Keep in mind that when transitioning from conventional shampooing, it may take a couple weeks for hair to adjust. I also recommend the “Definitive Guide to Washing Hair with Dr. Bronner’s” written by my colleague, Rafi. It’s a great trouble-shooting guide in itself, and the comments are full of good tips too. https://www.drbronner.com/all-one-blog/2017/03/definitive-guide-washing-hair-dr-bronners/
Hi .. I was truly worried about the PH of soaps for my face, as I am 70, and no longer wish to use cleansers with all those chemical. I only started using goat milk soap this week, really like it and was worried about Ph. The goat milk on its own has a ph of 5.5 like skin, but in the final bar product, the Ph goes to 6 to 10 depending on the bar. Having read your article, I am happy to continue using bar soap on my face. I also wanted to mention .. citric acid does not lower PH. It is there as a chelator to bind with unwanted substances like calcium etc, to make sure soap does not oxidize or become rancid and have a scummy effect. I also use Dr . Bronners in a foamer bottle on my body and really like it ! thank you for your research !
Hi NC- I’m glad you found this article helpful! Citric acid is certainly a versatile ingredient! We use it to make sure that there are no unreacted sodium or potassium hydroxide ions leftover from the saponification reaction that turns the oils into soap. We also add tocopherols (derived from sunflower oil) as an antioxidant to extend the shelf life of the soap. Gotta love chemistry!
Can I use castille soap on the vulva? Those the following quote apply to the vulva area as well?
“Happily, though, the acid mantle is in a constant state of regeneration and returns to its normal pH within 30-90 minutes. The temporary pH disruption is not a concern.[v] Even long-term use of soap does not impact the pH of the acid mantle.[vi]”
Hi Sun- Yes, the pH of the Castile soap is fine for washing sensitive areas.
As a medical doctor i advice you please do not wash your vulva area with any kind of soap. All you need is water for that area. Using soap would change the microflora in that area leading to opportunistic infections like vaginal thrush
I have the African black soap similar to the Castile soap, which on submission to have CT/PET for microbial contamination they are insisting I pour preservatives because the liquid soap contains water.
My dilemma is I cannot find a preservative that word with a high pH of 9+
What’s your views on this?
Hi Reg- We’ve chosen to go with an antioxidant instead of a preservative. We use tocopherols derived from sunflower oils. They work well even with the alkaline pH of the soaps.
Thank you, Lisa, for your kind attention. I have observed that it doesn’t seem to be an issue for my husband either. In the future I may get him some Dr. Bronner’s hair rinse, present circumstances just don’t allow me to do it yet.
Dear Lisa, I have been enjoying trying both the liquid castile soaps and shaving soap for hair washing and I do a diluted ACV rinse. However, I will never get my husband to bother to use an ACV rinse, so my question is: Is there a way for me to make the castile soap more acidic so that he doesn’t need to do a special rinse? If you have any other suggestions, they will be welcome. Thank you.
Hi Shirley- Both my husband and son use the soap to wash their hair, and neither one bothers with the acidic rinse. There is no way to make the soap more acidic. Adding an acid will cause the soap to unsaponify.
To Shirley,
I found a recipe for shampoo using Dr. Bronner‘s Castile soap. You add coconut milk and some other oils. See the recipe on six dollar family.com/homemade coconut shampoo.
Thank you for sharing, Nena! Bear in mind that adding oils to Castile soap reduces its cleansing ability on scalp and hair. The soap molecules will instead go after the coconut milk and oil molecules. What does and doesn’t mix with Castile soap is covered over here on my blog: https://www.lisabronner.com/what-can-you-mix-with-castile-soap/
Lisa,
I ‘m a 62 year old and love, love the products. I will be mentioning it in my blogs for women of color 60 and older. I do have a question, does it bleach or tone the skin?
Thanks,
Hi Janice- It’s great to hear you love our products! None of our soaps will help to reduce dark spots or lighten skin. Castile soap does provide skin with what it needs to strong and healthy though.
Thank you so much for this article and for the references. I can now make educated choices when it comes to my skin care.
Lisa, for hand washing we use foaming pumps with distilled water and Unscented bronners.. what would happen if we added lemon juice or lemon essential oil to the mix? Would this acid bring down the ph closer to neutral and therefore less disruptive to the mantle? I know you mentioned the shikakai that brought down the alkalinity in the shaving soaps. I’m wondering if there’s any acidic substance that might be beneficial to add. Not sure what this could do to the chemical composition or effectiveness of the soap. Thanks for your diligent research.
Hi Ali- Lemon essential oil is cold-pressed from the rind of the fruit and is pH is neutral. It would have no chemical reaction with the soap (but would create a fresh new scent!). On the other hand, citrus juice comes from the pulp of the fruit and is acidic. Any acid, be it lemon juice or vinegar, is going to cause the soap to unsaponify and cause a goopy mess.
Honestly, there is no one on the market who understands soaps like you Dr Bronner. I love the work you do, I love that you made this so simple for anyone to understand, even though you had to read tonnes of complicated research. I love your soaps and your brand in general. Thank you so so much for this. I am no longer scared of ph!!! Sharing this article with the world!!
Thanks for the love, Crystal!
[…] There’s a lot more to the discussion of pH and its role in skincare in my post Skin Health, pH, and Dr. Bronner’s Soap. […]
[…] change, concerned about pH values and sensitive skin. I found a great article from Lisa Bronner – Skin Health, pH, and Dr. Bronner’s Soap. She does the science and even water alters the pH balance when you wash your hands. We tested out […]
Thank you for this research and simple explanations! I haven’t tried your products yet but I think that they are of the highest quality. Can’t wait to try your famous soap!
Hi Joshua- We can’t wait for you to try it either!
I am so glad you did this research! This all makes sense to me now, as I have been delving deeper into what I put on my skin. I am in my 60s and have dry skin. I have been using Dr. Bronner‘s Castile soap and making shampoo out of it also. My hair and skin feel very soft.
Hi Nena- It’s great to hear you found the article helpful! Thanks for your support!
Thanks so much for this thorough research Lisa! This answers so many questions I had and although I have done my own research in the past, I have never seen anything this clear and easy to understand summary! I am going to have this translated so our fans in Korea can learn about this too – you rock Lisa!
Hi Grace- It’s great to hear from you! Glad the article helps!
Thank you so much for this helpful information, with lots of different cleansing products in the market it is difficult to determine which one is best, I trust your products I have been using them for years now
Hi Nen- Yes, a trip down the skincare aisle of any store is enough to get your head spinning! Glad you found the information – and our soaps – helpful.
Is one cleanser better for sensitive, rocesia, easily, but has black heads, dry, irritated skin than another?
Hi Cheryl- As you probably know, there are a lot of things that can trigger rosacea. Because it is partially an inflammatory reaction, the Tea Tree Castile is the best option. Tea Tree essential oil has natural anti-inflammatory properties. This will not prevent flare-ups, but can help soothe them. Tea Tree is also good for blackheads. If you find this is too strong, try the Unscented Castile soap.
Great Article!!! Thank you for sharing – I really appreciate every word & every detail. For once I dont feel like Im left with more questions than answers!!!!!
Hi Tami- That’s great to hear! I’m glad the article was helpful to you.
[…] of tolerable pH, around 7.54+/- 0.01. This is ever so slightly alkaline (a pH of 7 is neutral). Dr. Bronner’s Castile soaps are around 9.3, a pH not at all irritating to our skin. To lower the pH would neutralize the cleaning ability of […]
[…] Bronner’s Castile soap and Sal Suds are perfect for this because they are both alkaline. This is the opposite of acidic. An acid can etch a soft stone, but an alkaline won’t. On […]
Dear Lisa Bronner,
THANK YOU FOR ALL YOUR RESEARCH AND TIME…VALUED IN AN ARTICLE SO WELL WRITTEN AND VERY UNDERSTANDABLE. YOU Broke down all the Science in Simple yet readable way that Made Sense..I personally been Struggling for YEARS, I was diagnosed with Heavy Metal Poisoning many years ago and while going through the chelation process the doctor told me to expect a heightened allergy to certain foods and petroleum products. This has meant an OverHaul of getting RID of all Cleansers: bar and liquid soaps, shampoos.deodorants, laundry detergents, fabric softeners.ETC..
The doctor said I would never get all the toxic metals out of my body But by eliminating the toxicity of outside influences as mentioned above plus a Proper diet of whole foods100% Plant Based as Food from Our planet Provide so many benefits and Fiber that naturally pull toxicity and heavy metals that poison me. I now Use natural cleaners such as Your Wonderful liquid Castile soaps as my body soap, shampoo and general household cleaner…Thank You!!!… And Keep Making Your Family Soap…Because its is SO Happy on my Skin as Now I Don’t have PAINFUL RASHES (all Due to Petroleum Products) Covering all over my body. ..Thank You Again!
Sincerely
Cynthia Walker.
Hi Cynthia- I’m glad to hear of your return to good health! It’s nice to hear our soaps were part of your journey to get there. Thank you for sharing your story!
Thanks for doing the research and explaining it in a way laymen can make sense of. I will be sharing this piece wildly. Could you at some point do a similar piece on the unnecessary addition of “fragrance” that so many other soap makers use? I use Dr. Bronner products for just about everything: wash my hair with the sugar soap instead of buying Shikai branded products with too many ingredients; use leave in Lavender Coconut hair conditioner – I still have a full head of wavy salt and pepper hair at 67 years of age; wash my clothes with Lavender liquid soap, white vinegar instead of fabric softener, and a cup of baking soda dissolved in 3 cups water poured right on the clothes before closing the door (if anything needs bleach I use hydrogen peroxide); and in the past year started using the toothpaste. Our laundry area is just basically a closet with stackable washer dryer combo and could at times get a funky smell, but not when I use the lavender soap. And I can’t forget that indispensable item for cold northeast winters: Patchouli Lime Body Balm. It works wonders, especially after washing a lot of dishes.
I look forward to your next informative entry.
Hi Michael- Glad you’re aware of the issues around the term “fragrance.” I agree, it’s a topic that needs addressing and its on my list as one to dive into in a blog post later this year. Keep your eyes peeled for it. Thanks for being an ardent user of our soaps!
Thanks so much for this informative and insightful article.
I am an Industrial Chemist and a soap maker. I just made my first successful batch of castile soap and I was bothered about the pH. This article resolved a lot of the issues bothering me.
Thank you.
Do I have to dilute the bar form of the soap in water to use on my hair?
Hi Makayla- We suggesting working the soap into a lather in your wet hands before applying to hair and scalp. That’s all the diluting it needs.
Nicely written article, full of all the science facts that we nerds like to know about prior to purchasing body care products. I’ve been using both Bonner’s bar and liquid soaps for several years. An acquaintance of mine has a dermatologist husband who would never recommend anything else to his patients with sensitive, dry skin (and probably problem, acne skin, but that wasn’t my issue at the time, so therefore, never got his opinion on that). So, I pose my question to you: my daughter has very problematic (acne) skin that is very resistant to diet change, special products, antibiotics, creams and gels. We are slowly, but surely gaining the upper hand on this stubborn problem, but my desire is to have her OFF all these things that can adversely affect her body in other ways. What Bronner product would you recommend to help clear up stubborn all-over face, chest and back acne? A castille soap or a sugar soap? Perhaps the tea tree oil? Thank you so much to you and your family to dedicating your lives to providing others with the care and commitment you steadfastly provide!
Hi Kelly- I’m glad our soaps have been helpful to you! Acne is a challenge at any age, but it’s especially difficult for a young person. It sometimes can feel like quite a puzzle to figure out what works for whom because acne has many different causes – sometimes it is because of excessive oil production, sometimes because skin is too dry, sometimes it is hormonal (e.g. adolescence, monthly cycles), or a variety of other reasons. Regardless, I’m glad to hear you and your daughter are getting the upper hand. Tea Tree Castile helps treat acne-prone skin and balances out oily or combination skin. The organic sugar and white grape juice in our Sugar Soaps are natural humectants and draw moisture into the skin, and would be an option if your daughter’s skin is dry. Because shampoo can also cause acne on the chest and back, consider having her try the Castile soap in place of conventional shampoos. You can read more about that here: https://www.lisabronner.com/from-shampoo-to-soap-%E2%80%93-my-story/.
Wow! Excellent and thorough research that further solidifies my support and use of Dr. Bronners products! Thank you!
Hi Ann- That’s great to hear! Thanks for your support.
Thank you, Lisa, for all that you do and for staying on top of things and making science so interesting and that makes sense. I appreciate all you do and love all your great products and education!
Thank you, Rosalee. I’m glad you find my blog helpful and interesting!
I’ve been committed to Bronner’s soaps for a long time and that question was always in the back of mind. I LOVE to know the research. Thank you so much for writing this. Didn’t think I could love you all any more than I did but I do now!!
Hi Marie- It was always in the back of my mind too. It was daunting to dive into all the research, but I’m glad I did. Thanks for following along and appreciating the data!
Thanks for the homework and providing a great education on the health of the skin. These days the care and feeding of the bacteria in and on our bodies is receiving more attention. Did you come across anything useful regarding the skin biome and what we need to do to support the good bacteria and remove the bad?
Hi Guy- The skin biome didn’t come up in my research for this topic, but it is an important part of the skin’s ecosystem. Washing does temporarily affect the skin biome, but our bodies can replenish it easily as long as we stay away from overly harsh cleansers and don’t over-wash.
Wow! This must have taken hours and hours of research. It is absolutely outstanding. I didn’t learn this much when I took chemistry in school! I love Dr. Bronner’s soaps!
Hi Michael – While I enjoyed high school chemistry immensely, I had similar thoughts when I was in the thick of my research.
Very interesting! Thank you!
One question I did have about the pH and DIY foaming handsoap. I read on a few sites that in order for it to be pH balanced and to not grow any mold or bacteria, you need to add a preservative. Would that be the case with using your liquid castille soap? Also, those sites said to use the diy soap within a week… I’ve been making it and using it without a preservative, just your castille liquid mixed with distilled water and some essential oils, and using it til it’s gone, definitely over a week! What are you thoughts?
Hi Hannah- Your nose will tell you when it’s time to toss your dilution. We say diluted soap has a shelf life of a month, and while it gets used up quickly at my house, I’ve had some batches for longer without going bad.
Ok, thank you! I use a smaller pump, so it is gone by a month!
Do you need to add a preservative or is pH and everything else good to go with just mixing your castille soap with distilled water, and essential oils?
Hi Hannah – I have never added a preservative to DIY solutions. The Castile soap is naturally preserved because of its alkalinity, and we also add Tocopherols (vitamin E) as an extra preservative. That will keep most dilutions safe for as long as it would take you to use them up. Using filtered or distilled water would not immediately introduce bacteria into your solution, but rather the issue is that bacteria in the air might eventually get into the solution and there wouldn’t be enough preservative to combat it. All that being said, your solution is safe for a while, but if you wanted to, you could add a couple drops of vitamin E. If you notice that it is smelling off, then dump and remake.
Thank you so much!! Love all that you and the company do!!
Hi, Lisa. You are wonderful for the time you have taken for explaining about the PH mantles of our skin. I do have a personal question when it comes to the PH of Womens feminine areas. Can we use all of Dr. Bronners soaps, liquids as well as the bars, in those mentioned areas( especially pertaining to the peppermint and stronger essences). Will it keep those areas PH neutral-balanced? Thanks so much !
Hi Ruth- The body’s pH varies by area and is higher in areas with more moisture, such as under the arms and areas “down under.” Any washing is going to disrupt the pH, but it will return to normal within an hour or two. The key is to use a mild soap and not wash too often. Dr. Bronner’s soaps are safe all over, although the scents with a milder essential oil, such as Almond, Citrus, Rose or Unscented are recommended for more sensitive areas.
Thank you for doing the research. This is just what I needed to get started with your products. I have noticed that my hands are a little dry after using it as a hand soap. Is there any sort of solution for this? Anything I could safely add to my hand soap?
Hi Deb- Welcome to the family! Frequent hand washing can certainly do a number on one’s hands, especially during these winter months. Some of the essential oils in our soaps, such as Peppermint and Eucalyptus can be more drying than others. Opt for a more gentle scent like Rose, Citrus or Almond, or our Unscented, which has no essential oils and double the olive oil. The Unscented Magic Balm helps heal and protect hands as well. If your skin is on the dry side, you may like our Sugar Pump Soap. It contains natural humectants that nourish skin and draw in moisture. The Castile soap is exceedingly versatile beyond hand washing. Refer to the cheat sheet for its many uses: https://www.lisabronner.com/dilutions-cheat-sheet-for-dr-bronners-castile-soap/
Thank You, Lisa, for another interesting, science-based article to put minds at ease. It is always nice to see you care. Love your products and enjoy the “recipes” you have shared for cleaning solutions. Cheers.
Hi Margarita- It’s great to hear you find my blog helpful! Thanks!
Thank you I love your soaps ? and all your products ?
Hi Valerie – Thank you! I’m glad this was helpful!
Your commitment to the health of your customers is really encouraging. I’d done independent research into the same issue and came to the same conclusions, but to have that honest investigation done by a member of the Bronner family themselves, on behalf of customers with less time than me, is really something you don’t see from companies with less integrity (e.g. nearly all of them).
My most recent addition to my skin care is your peppermint lotion. I’ve seen complaints that it’s a little thin, and that is both true and what I love about it! It’s perfect for a light rub-in after washing my hands. I always feel a little dry after washing, regardless of the soap used, and the lightness of your lotions are perfect for replacing that moisture without the heaviness of what I use in the morning (sadly not a Bronner’s product; I hope you’ll consider releasing a heavier, all-day lotion as well).
Hi John – Thanks so much! I’m glad we’re in sync on this. And I’m glad you found a place in your heart for the Peppermint Lotion! I love it, too, especially in the summer. I don’t need a heavy lotion, so the lightness is perfect, and a bit of that on the back of my neck, with the cooling peppermint oil, is the perfect pick-me-up for the afternoon doldrums at my desk. I hear you, though, about a heavier all day lotion. It’s on our radar. We don’t move quickly because we want to make sure we get things right, but it’s on our radar. (Our newest product – launching one week from today – is a new hand sanitizer scent! Exciting stuff! I can’t give away which scent, but check it out next week.)
Another excellent read Lisa! So many companies ‘think’ they are deciphering all the mumbo jumbo by trying to emphasise what’s NOT in their products, instead of what is! Dr. Bronner (& co.) tell it how it is. Your blog really does make Sense of Science!
Hi James- It’s great to hear you found the article helpful. The science of soap is good stuff!
WOW!!! Nice, nice work!!! You answered questions I’ve always had (especially about Bronner’s, because I really love the products!). YAY and FULL SUDS ahead!!
Hi Janis- Glad your questions have been answered! Full Suds ahead – Ha!
Hip Hip!! Maybe you are on your way to an advanced degree now Lisa?? Thank you from a fellow Word Nerd ?
Word Nerds unite! Thanks for reading, Corinna!
Excellent and very interesting !! Thank you for taking the time to thoroughly research the subject and share it with all of us. Much appreciated….
Hi Susan- Thanks for reading!